COVID-19 creates opportunities for international research
Ryan Noone
Jun 24, 2021
Source: Department of Chemical Engineering
As a result of the COVID-19 pandemic, universities worldwide have been forced to reduce in-person interactions, altering how we connect and work with one another. The transition to a “virtual world” has been eye-opening, proving to many that research and innovation can continue, even in a remote setting.
For Kris Dahl, professor of chemical engineering at Carnegie Mellon, the pandemic has in part created both international and interdisciplinary research opportunities. In conjunction with the CMU Portugal Program, Dahl teamed up with João Sanches, associate professor at the Institute for Systems and Robotics (ISR), to co-advise Portuguese master’s student Maria Teresa Parreira on the analysis of data obtained during a study considering cellular monolayers of epithelium.
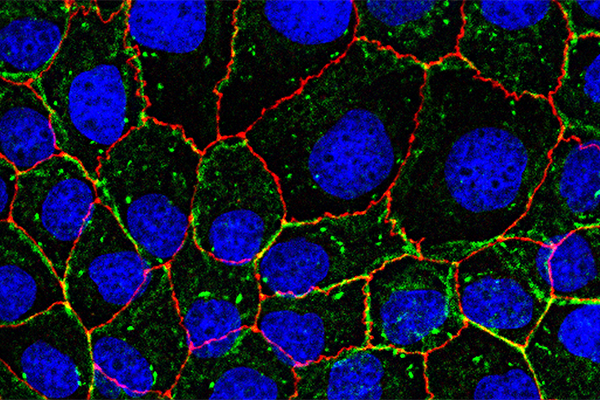
Epithelial layers line most of the exposed regions in our bodies, such as the lungs, digestive tract, genital tract, and to some degree, even our skin. When cells in the epithelial layer stiffen, it is often a sign that they have become cancerous or fibrotic. In a lab at CMU, Dahl and her group stiffened single cells within epithelial monolayers and examined the morphology and motion of surrounding cells. The data was collected in the form of images, then sent to Sanches and Parreira, who conducted morphometric analysis and temporal tracking to determine the results.
“We’ve always analyzed our data with respect to standard analysis techniques, but João and his team were able to show us brand-new techniques that we hadn’t used before,” said Dahl. “Everything was automated, and they had some very clever ideas on how to analyze the shape of these cells over time.”
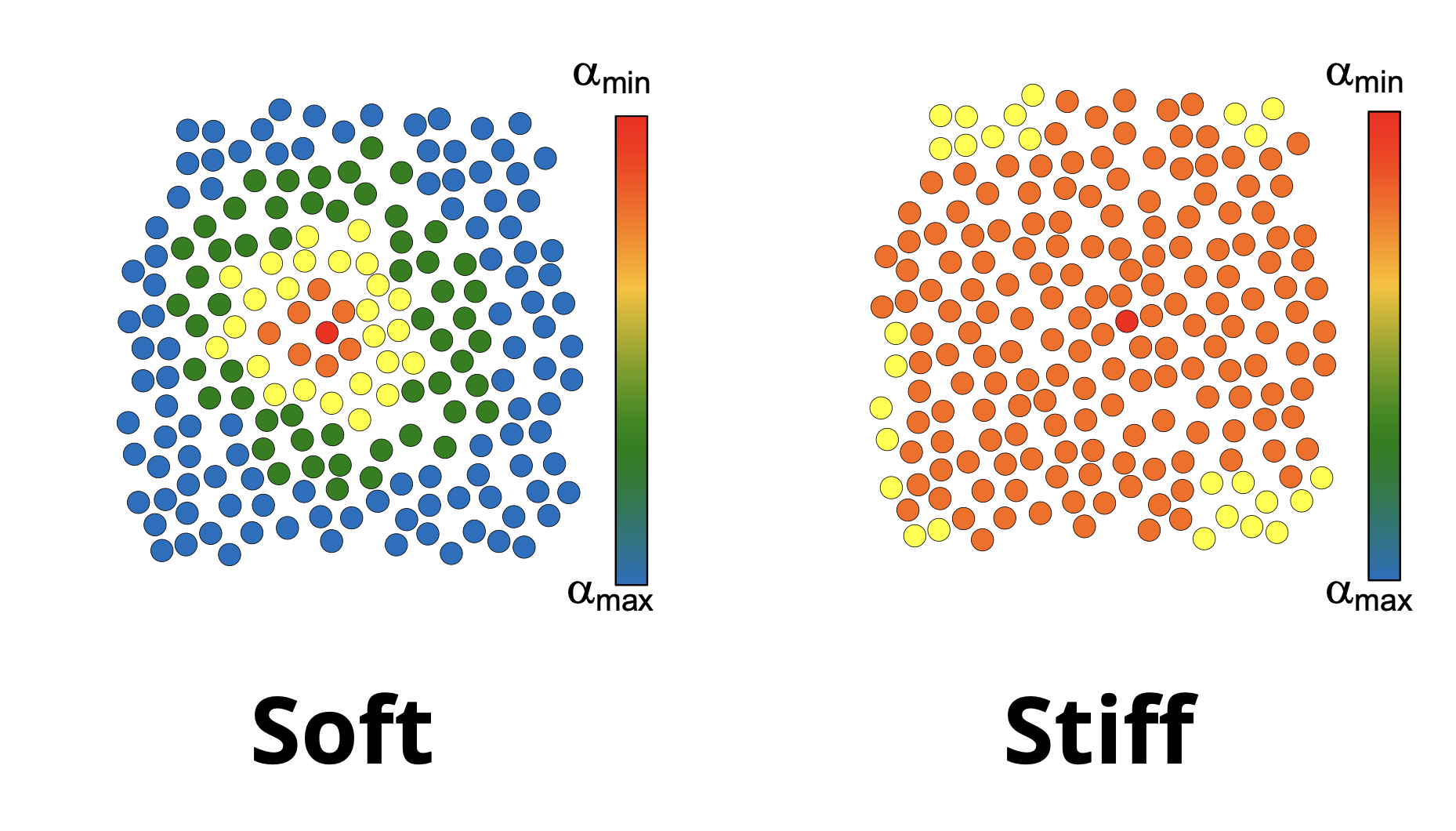
Predictably, the stiffened cell was significantly altered; however, Dahl was surprised to find drastic changes in the cells radially outward from the stiff cell. The study revealed that cells are highly sensitive to the presence of mechanically impaired neighbors, leading to a generalized loss of coordination in collective cell migration. Normal cells within the monolayer appear to be dependent on the distance to the impaired cells, pointing to compensatory behavior in response to force transmission imbalances in the monolayer. The results suggest long-range impacts of single-cell defects related to tissue dysfunction and provide insight into how fibrosis can start and become so devastating in different body tissues.
Source: Department of Chemical Engineering
In a previous study where Dahl softened cells within epithelial monolayers, surrounding cells appeared unfazed. However, in the current study, drastic changes were recorded in cells radially outward from the stiffened cell.
“Many areas of the body have the ability to regenerate, but these areas lose that ability once they become fibrotic,” said Dahl. “By understanding how fibrosis is initiated, we can work on developing ways to interrupt that process.”
Dahl will present the team’s research at the upcoming European Society for Clinical Hemorheology and Microcirculation, International Society of Clinical Hemorheology, and International Society of Biorheology Joint Meeting on July 4-7, 2021.
In the future, Dahl hopes to build upon this research by collaborating with the University of Pittsburgh Medical Center (UPMC) to understand how cells react to stents placed in the body. When a patient experiences coronary artery issues, physicians perform a procedure called angioplasty to open narrow or blocked blood vessels that supply blood to the heart. Immediately following the surgery, a stiff metal mesh tube known as a stent is placed inside the blood vessel to keep it open, allowing blood to continue flowing. Due to a stent’s stiff properties, there is reason to believe they may significantly impact surrounding cells.
This paper was published in Cytoskeleton in 2021. Other authors include Kirill Lavrenyuk, doctoral student in Carnegie Mellon and the University of Pittsburgh’s joint Molecular Biophysics and Structural Biology Program.
