From fridge to lunchbox: Measuring sulfuric acid in the air
Lauren Smith
Mar 20, 2024
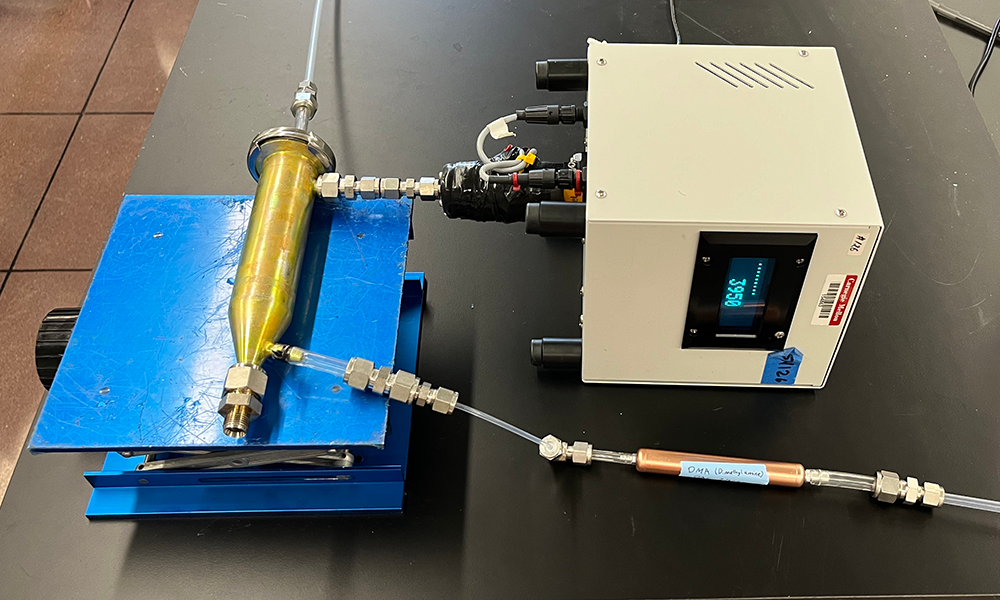
Source: Coty Jen
The prototype of the sulfuric acid dimethylamine-reactive condensation particle counter in the Jen Lab.
Climate scientists have long known that sulfuric acid, the major contributor towards acid rain, is in the air, but they haven't been able to monitor it. Instruments traditionally used to measure sulfuric acid are very expensive, power-intensive, and large.
Coty Jen is developing a smaller and less expensive instrument to measure ambient sulfuric acid in the air. Jen, an assistant professor of chemical engineering, received a Department of Energy Small Business Innovation Research grant in collaboration with Aerosol Dynamics, Inc. They are partnering to commercialize the instrument, named the sulfuric acid dimethylamine-reactive condensation particle counter (SAD-RCPC). Jen has also submitted a patent application.
The Jen Lab originally designed the instrument to help understand how sulfuric acid is driving the formation of atmospheric particles in cities. Sulfuric acid is a terminal product of the sulfur dioxide released from burning fossil fuels. In cities, where more people burn more fuels, high concentrations of gaseous sulfuric acid can result in sulfate contributing up to 30% of aerosol particulate mass.
It's unclear, however, if sulfuric acid is well distributed around cities, because measuring this compound in the air is difficult and expensive. This is also a reason why there are currently no regulations for sulfuric acid in the air. Like with particulate matter (PM), including PM2.5 and PM10, better measurements lead to more impactful regulations.
Illustration of the type of urban map that could be created with measurements from the instrument. The red, yellow, and green dots indicate hypothetical high, medium, and low concentrations of ambient sulfuric acid in the air.
Commercializing the SAD-RCPC will get the instrument into more labs. "This will enable the research community to start monitoring urban sulfuric acid concentrations more widely," says Jen.
In May, Jen's research group will travel to Oklahoma to test their prototype on a tethered balloon from the Department of Energy. The small size of their instrument is key to this test. Traditional instruments are approximately the size of a refrigerator. "You can't put a fridge on a balloon," says Jen.
Based on the working prototype built in her lab, Jen's goal is for the instrument to be the size of a lunchbox and ten times cheaper than what is currently available.
The Jen Lab will also demonstrate cost-effectiveness and accessibility as part of another project. Passenger ferries in the Gulf of Maine will carry the instrument to take measurements of the air over the open ocean. Again, the size of the instrument makes this possible. Most research instruments require a very large boat, which makes the costs of fieldwork very high. Using pre-existing boat routes, like the Jen Lab will do in the Gulf of Maine, lowers the costs.
With the grant, the Jen Lab will package the instrument and make it more user-friendly. They have one year to demonstrate that their instrument will work at its smaller size and in cities, before applying for the second phase of funding for continued commercialization.
For media inquiries, please contact Lauren Smith at lsmith2@andrew.cmu.edu.
