Optimizing the recovery of rare earth elements
Lauren Smith
May 13, 2025

As demand for rare earth elements increases, the United States is strengthening its domestic supply chain. Ana Inés Torres is working to identify economical processes that will minimize environmental impacts.
Her findings show that recovering rare earth elements from end-of-life products is a viable approach. It is more profitable than extracting rare earth elements from ores, where they are diluted and also mixed with radioactive materials, and it keeps rare earth elements out of landfills.
Rare earth elements encompass the lanthanide series, scandium, and yttrium. Their strong magnetic properties make them essential to clean energy technologies.
Electrification will require a reliable supply of rare earth elements. One wind turbine, for example, uses more than two tons of rare earth elements.
Torres, assistant professor of chemical engineering, has developed an optimization-based framework to design the best process for recovering rare earth elements from end-of-life products. The model is informed by estimates of projected quantities of end-of-life hard disk drives and electric and hybrid electric vehicle motors, as well as by projected prices of rare earth oxides.
Source: Chris Laliwala
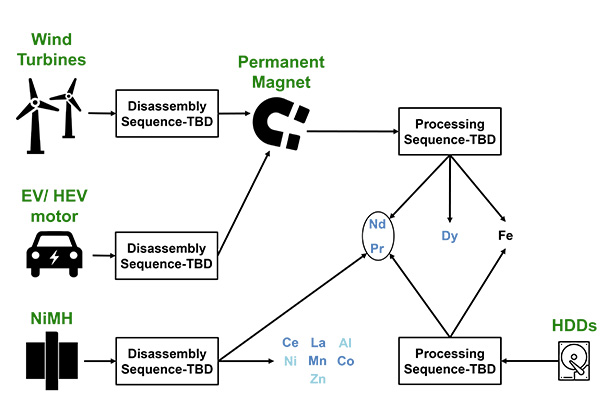
Recycling rare earth elements from end-of-life products typically involves four stages: disassembly, demagnetization, leaching, and extraction. Torres and Chris Laliwala constructed a superstructure containing all possible processing pathways. There are several potential processes within each stage, which are represented as nodes in the superstructure.
They then formulated an optimization problem with the goal to maximize net present value over the 15-year lifetime of a processing plant. Lastly, they conducted a sensitivity analysis of the optimal pathway to determine the impact of changing different parameters.
The Torres Research Group is actively partnering with industry to improve the processes they have identified as optimal and to make them more environmentally friendly. "One company we are working with has a pilot plant that they are scaling up to industrial production," says Torres. "We're not going to be waiting for years to see these processes used in the real world. It's already happening."
We’re not going to be waiting for years to see these processes used in the real world. It’s already happening.
Ana Inés Torres, Assistant Professor, Chemical Engineering
With each new application of superstructure optimization, the Torres Research Group must introduce new considerations into the problem. The supply of end-of-life products, for example, is not in a steady state. Laliwala, a Ph.D. student in chemical engineering, had to modify the math behind the superstructure to account for a changing supply from year to year.
Superstructure optimization techniques are not widely used because they involve a lot of time-intensive coding. To make them more accessible, the Torres Research Group is collaborating with Lawrence Berkeley National Laboratory to develop a graphical user interface. The program will write code and run the optimization based on the selected processing alternatives. "This will democratize the use of these tools," says Torres. "A chemical engineer or an experimental chemist will be easily able to compare their new advances with the processes that we have already studied."
Torres is also collaborating with colleagues in the Department of Materials Science and Engineering to use rare earth oxides in advanced additively manufactured structural alloys that can sustain extreme environments. The Torres Research Group is simulating processes to convert rare earth ores into rare earth oxides.
Their optimization-based approach models rare earth recovery systems as chemical equilibrium problems. They are applying it to simulate and assess conventional methods for extracting rare earth elements. These processes were used when the US dominated the rare earth elements industry 40 years ago. They were considered costly and a cause of significant environmental damage. "Our goal is to design processes that will be environmentally friendly and economically competitive, but first we need to understand where past bottlenecks were," says Torres.
The Torres Research Group is working to understand the effect of each processing step. With that information, they will synthesize more economical and environmentally friendly processes to convert rare earth ores to commercial-grade rare earth compounds. Their findings can help US policymakers choose the best paths to restoring the processing of rare earth elements in the US.
For media inquiries, please contact Lauren Smith at lsmith2@andrew.cmu.edu.
