A mathematical picture of properties at fluid-fluid interfaces
Lauren Smith
Oct 7, 2025

In cosmetics, food science, drug delivery, and agrochemicals, industrial formulations mix one type of fluid in another. Researchers in the Department of Chemical Engineering at Carnegie Mellon University are working with The Dow Chemical Company to explore what happens at interfaces between fluids.
Interfacial properties like surface tension affect processing. "Surface tension tells you, for instance, how vigorous of a flow you have to apply to get the material to break up into an emulsion," says Aditya Khair.
Khair, a professor of chemical engineering, is collaborating with research scientists at Dow to understand the effect of molecules like surfactants on surface tension. Surfactants have hydrophobic and hydrophilic parts. This characteristic naturally attracts them to oil/water interfaces, where they decrease surface tension.
Some surfactants are ionic and possess an electrical charge, which can alter the chemical environment surrounding the fluid interface. "All these molecules want to collect at the interface, but they don't want to be next to each other because they're the same charge," says Emerson Uhlig, a Ph.D. student in chemical engineering. For their initial work with Dow, Khair and Uhlig wanted to be able to predict how these ionic surfactants affect the surface tension as they move to a fluid-fluid interface.
In the Journal of Colloid and Interface Science, Khair and Uhlig paint a mathematical picture of what the interface looks like. It is an electrical double-layer, consisting of surfactant molecules and their charges, followed by the counter ions that they dissociate from and leave behind.
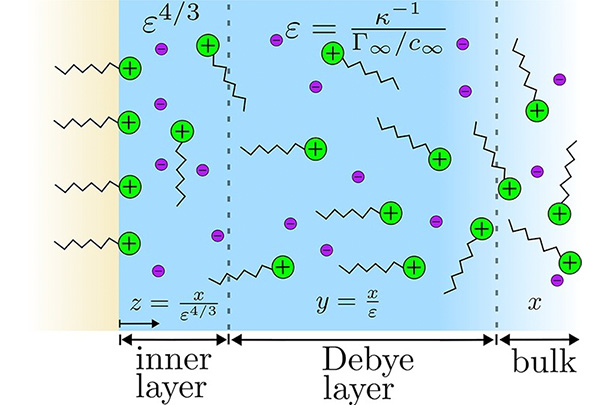
Source: Journal of Colloid and Interface Science, https://doi.org/10.1016/j.jcis.2025.137924
Khair and Uhlig used mathematical methods to simplify the problem of predicting the surface tension. They first determined how much of the surfactant goes to the interface. Because an ionic surfactant leaves oppositely charged ions in the fluid behind it, they then determined how those ions arrange next to the interface. "You can mathematically cast that into a set of equations," says Khair.
Khair and Uhlig's mathematical analysis quantifies how the double-layer structure impacts surface tension. "We provided new predictions that tell us how the interfacial tension is lowered by ionic surfactants," says Uhlig.
Their approach can be used in industry to determine how much surfactant is needed in a formulation to get a desired surface tension. The method is more efficient than the current standard because it does not require expensive computer simulations.
Solving problems by simplifying them to their minimal ingredients is a hallmark of Khair's research. He always looks for the essential physics.
Product formulations are complex, and the problems companies encounter are often more complicated than the ideal scenarios found in the literature. "Part of our job as collaborators is to identify the critical parts of a process or a formulation that's leading to the issues," says Khair. "We get to dissect these industrially-relevant problems," adds Uhlig. "We have to sort through the information in order to draw a consistent line and find the fundamental problem that may give guidance about the more complex issue."
For media inquiries, please contact Lauren Smith at lsmith2@andrew.cmu.edu.
